Introduction: CARTITUDE-4 is a phase 3 randomized controlled trial (RCT) assessing the efficacy and safety of CARVYKTI (ciltacabtagene autoleucel; cilta-cel) versus pomalidomide, bortezomib and dexamethasone (PVd) or daratumumab, pomalidomide and dexamethasone (DPd) in patients with relapsed and refractory multiple myeloma (RRMM) who have received one to three prior line(s) of therapy that included an immunomodulatory agent (IMiD) and a proteasome inhibitor (PI), and who are refractory to lenalidomide. CARTITUDE-4 demonstrated superiority of cilta-cel over PVd and DPd on progression-free survival (PFS) and response rates (overall response rate [ORR], very good partial response [VGPR] or better, and complete response [CR] or better [CR or stringent CR]). Other available therapies for the treatment of this patient population where individual patient data was available for analysis include: daratumumab in combination with bortezomib and dexamethasone (DVd), bortezomib in combination with dexamethasone (Vd), carfilzomib in combination with daratumumab and dexamethasone (DKd) or dexamethasone alone (Kd), and pomalidomide in combination with dexamethasone (Pd). This analysis aims to compare efficacy outcomes in patients randomized to the cilta-cel arm (i.e., patients apheresed or infused with cilta-cel) in CARTITUDE-4 vs each of these regimens in the same target population.
Methods: Individual patient data were collected from the CARTITUDE-4 (cilta-cel), CASTOR (DVd and Vd), CANDOR (DKd and Kd), and APOLLO (Pd) RCTs. Patients who met CARTITUDE-4 eligibility criteria were identified from the above mentioned RCTs. As no patients in the comparative trials had prior treatment with anti-CD38, patients with prior exposure to anti-CD38 therapies in the cilta-cel cohort were excluded. Key prognostic baseline covariates including refractory status, ISS stage, presence of extramedullary disease, and time to progression on prior line, were adjusted for using inverse probability of treatment weighting (IPTW), with average treatment effect in the treated weights. Differences in baseline characteristics were assessed using standardized mean differences. PFS, ORR, VGPR or better, and CR or better were compared between patients in the cilta-cel arm and the comparator regimens. Relative efficacy of cilta-cel vs comparator regimens were estimated with hazard ratios (HRs) and 95% confidence intervals (CIs) from weighted Cox proportional hazards models for PFS, and response rate ratios (RRs) and 95% CIs from weighted logistic regression models for ORR, VGPR or better, and CR or better. Sensitivity analyses using alternative statistical approaches and including additional covariates were explored.
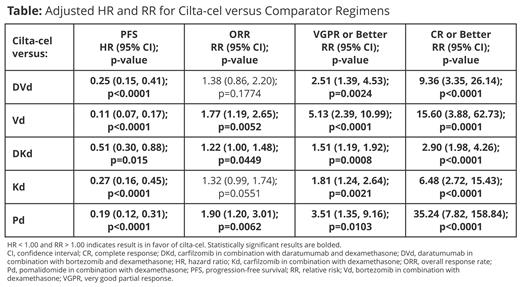
Results: After the exclusion of 53 patients with prior exposure to an anti-CD38 therapy, 155 patients in the cilta-cel arm remained. Across the comparative trials, 44 patients treated with DVd, 46 patients treated with Vd, 98 patients treated with DKd, 46 patients treated with Kd, and 92 patients treated with Pd met the inclusion criteria of CARTITUDE-4. Baseline covariates were similar across the cohorts after IPTW. Patients randomized to the cilta-cel arm showed significant improvements in PFS (adjusted HRs from 0.11 vs Vd to 0.51 vs DKd), VGPR or better (adjusted RRs from 1.51 vs DKd to 5.13 vs Vd), and CR or better rate (adjusted RRs from 2.90 vs DKd to 35.24 vs Pd) (Table). Improvements in ORR were also observed against all comparator regimens (adjusted RRs from 1.22 vs DKd to 1.90 vs Pd), though were marginally not statistically significant vs DVd and Kd. Treatment with DKd consistently yielded the best results out of all comparator regimens, but results were still in favor of cilta-cel. Results of cilta-cel benefit were consistent across sensitivity analyses, yielding adjusted HRs below 1.00 and RRs above 1.00 in all cases against other comparator regimens.
Conclusions: Data from CARTITUDE-4 and these analyses demonstrate the benefit of cilta-cel over additional regimens commonly used in clinical practice, highlighting its potential to become a new standard of care option for patients with RRMM, who have received one to three prior lines(s) of therapy, including an IMiD and a PI, and who are refractory to lenalidomide. These comparisons provide valuable information to contextualize the efficacy of CARVYKTI in countries where standard of care may be different from DPd/PVd.
Disclosures
Alsdorf:GSK: Honoraria; Jannsen: Honoraria, Other: Travel expenses; Biontech: Other: Travel expenses, Research Funding; Affimed: Research Funding. Diels:Janssen: Current Employment. Ghilotti:Janssen-Cilag SpA, Cologno Monzese, Italy: Current Employment. Mendes:Janssen Pharmaceuticals: Current Employment. Hernando:Janssen: Current Employment. Cost:Janssen: Current Employment, Current holder of stock options in a privately-held company. Schecter:Janssen: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Janssen. Lendvai:Janssen R&D: Current Employment, Current holder of stock options in a privately-held company. Patel:Legend Biotech, GSK, Freeline, BMS (spouse), AbbVie (spouse): Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Fernández de Larrea:Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal